BEATING LUNG CANCER
THROUGH EARLY DETECTION
LEAP is a non-invasive blood test to flag the presence of lung cancer.
Lung cancer usually starts growing deep in the lung and without symptoms. This “silent phase” presents a serious screening challenge and today 84% of new cases are late-detected.
Hawkeye Bio’s LEAP (Lung Enzymatic Activity Profiling) test is designed to safely detect cancer’s signature with a simple blood draw. Armed with this knowledge patients can obtain the timely medical attention they need to ensure the best treatment outcome.
LEAP is a simple blood test designed to detect enzyme activity related to lung cancer
The test presents no radiation risk and is 100% patient-safe
LEAP uses patented biosensor technology with 19 lung cancer biomarkers
Test results are fast and deliver high accuracy with very high sensitivity & specificity
A safe and easy test that requires only a blood sample.
Delivers a fast and actionable results.
Identifies early sign of cancer by detecting enzyme activity.
Performs with high accuracy; clinically validated.
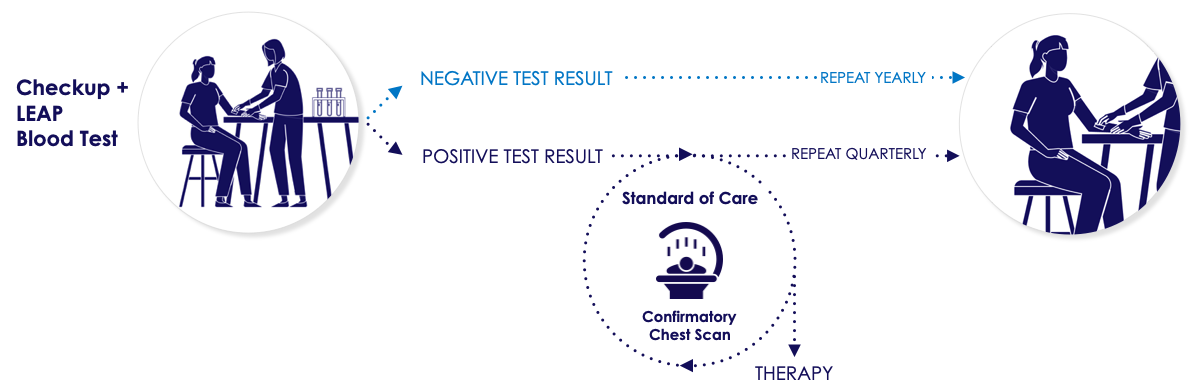
LEAP is designed to prescreen for lung cancer before it is conventionally discovered and confirmed by tissue biopsy.
With LEAP, people at risk for lung cancer can be cost-effectively identified and confirmed by LDCT so doctors can intervene before the metastatic process sets in, greatly improving the chances of survival. Because LEAP uses serum derived from a standard blood draw, it can also be used to complement current LDCT and liquid biopsy approaches by reducing the need for unnecessary biopsies.
Ordering the LEAP test.
The LEAP test is manufactured in California to the highest quality standards by Hawkeye’s FDA-registered, ISO 13485:2016-certified contract manufacturer. If you are a commercial lab, a medical professional, or an interested party the LEAP test is available through Hawkeye’s Early Access Program.
LEAP is available as a send-out test to Hawkeye’s CLIA lab.
LEAP is also available as a kitted test for commercial labs.